Abstract

Introduction: SPML is considered a hallmark of PV, but its frequency, as reported in the literature, ranges from 20% to 75%. It has been assumed, without documentation, that SPML may be accompanied by symptoms and signs and may affect prognosis. Moreover, expensive radiographic tests have become mandatory in some recent phase 2 drug trials in PV (and ET) to carefully document spleen size on trial entry and its change, if any, during therapy. Because systematic studies have not been performed, we studied SPML in patients (pts) with PV at their initial diagnosis (DX) or at first presentation (PRES) at our institution, Weill Cornell Medicine (WCM), and determined its clinical significance.
Methods: This single-center retrospective study was approved by the WCM institutional review board. A systematic literature search including PubMed, Embase, and Cochrane School relevant to the specific search questions was unrevealing. We used a research data repository based on an automated query system which had aggregated longitudinal clinical information pertaining to our PV patients for our analysis of spleen size (Abu-Zeinah et al. Leukemia 2021). Standardized PV diagnostic criteria were used for all pts (Silver et al. Blood 2013). As a tertiary referral center within a major metropolitan area, our PV population is composed of those diagnosed at WCM (DX) and those presenting to WCM some time after diagnosis (PRES). Degree of SPML was categorized into 3 subgroups: (1) <1 cm if the spleen was not enlarged, (2) palpable 1-5 cm, or (3) more than 5.0 cm below the left costal margin of the abdomen in the medial clavicular line in the supine or left lateral decubitus position; it was also considered enlarged after splenic ultrasound scan (US) based upon the method and verified formula of Chow KU, et al. Radiology 2016. Spleen size was correlated with age, sex, race, and ELN risk score, and symptoms including pruritus, night sweats, anorexia, abdominal discomfort and pain. Progression to myelofibrosis (MF) with myeloid metaplasia was defined per ELN/IWG-MRT criteria. Spleen measurements after progression to MF were excluded. Peripheral blood smears were routinely examined to view RBC morphology and to exclude leukoerythroblastosis. MF-free survival (MFS) and overall survival (OS) were calculated using the Kaplan-Meier log rank test among the various spleen subgroups. Multivariable survival analysis (MVA) was performed using a Cox proportional hazards model.
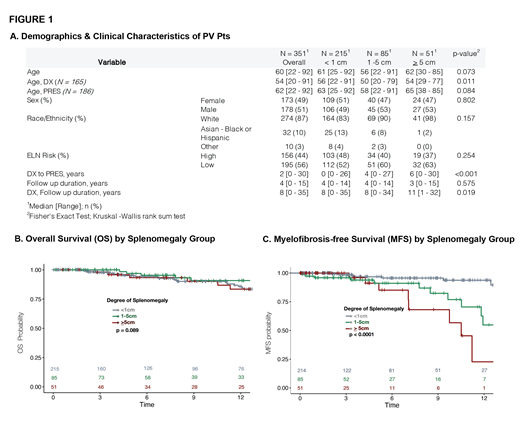
Results: From our 470 PV dataset, 351 pts had documented spleen size at DX (165) or PRES (186). The median age for all patients was 60 years (yr), for DX 54 yr, for PRES 62 yr. The median DX ages of SPML <1 cm, 1-5 cm, and >5 cm were 56, 50, and 54 yr respectively (p=0.011). The median time between first evaluation for PV and first visit at WCM (PRES) was 2 years (range 0-30). 49% were female and 13% were non-white (Figure 1a). There was no correlation between spleen size and ELN risk scores. The linkage between SPML and symptoms will be reported. Overall survival of the three groups was similar at 12 years (Figure 1b). SPML at presentation, however, was associated with increased risk of MF (1-5cm versus 0: HR 2.56, p=0.026; >=5cm versus 0: HR 5.64, p<0.001), independent of age or disease duration in MVA.
Discussion & Conclusion: Patients who had SPML > 5cm at presentation had a worse MFS than those with a lesser degree of SPML or no SPML (1-5 cm or <1 cm). For determining SPML, clinical examination and calculated US length were equally satisfactory. In the absence of clinical SPML, radiographic tests appear unnecessary. However, for SPML > 5cm, and unusual body types, more detailed radiographic studies may still be required. SPML was more common in younger patients, suggesting more aggressive disease and earlier progression to MF. ELN risk category did not correlate with SPML, suggesting an additional reason for its revision. Our PV patients with SPML > 5cm at DX or PRES did not have a decreased OS at 12 years, but did have a reduced MF-free survival. These data support the WHO mandated requirement for marrow biopsy for diagnosis of PV, especially for patients with SPML but also as a baseline requirement to establish the presence of MF less than grade 2. Patients with SPML> 5cm appear to be at high risk of MF progression and must be monitored closely for this event and treated appropriately.
Silver: Abbvie: Consultancy; PharamEssentia: Consultancy, Speakers Bureau. Scandura: CR&T (Foudation): Research Funding; European Leukemia net: Honoraria, Other: travel fees ; MPN-RF (Foundation): Research Funding; Constellation: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Abu-Zeinah: PharmaEssentia: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal